It is wonderful to see that a new stem cell treatment, using a patient’s own adult stem cells, is now being offered at the South Florida Bone Marrow & Stem Cell Transplant Institute in Boynton Beach, Florida. As you will see from this short video, the adult stem cell protocol is having dramatic, positive results for those suffering from Parkinson’s disease. If you know of anyone who has Parkinson’s Disease please pass this video on to them.
Archive for January, 2014
Adult Stem Cell Protocol for Parkinson’s Disease
Thursday, January 16th, 2014Adult Stem Cell Therapy for Spinal Cord Injuries
Friday, January 10th, 2014SAPPORO (Jiji Press)—Sapporo Medical University said Friday, January 10th, 2014 it will start Japan’s first clinical trial using stem cells for nerve regeneration in patients with spinal cord injuries.
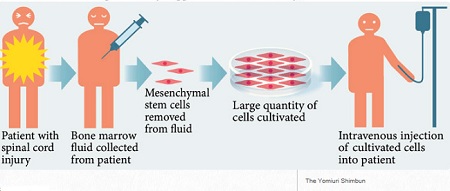
The team will collect bone marrow fluid from patients who injured their spinal cords within the previous two weeks, take mesenchymal stem cells (these being adult stem cells) that develop into nerves out of the fluid, and cultivate them in large quantities to make cell preparation for intravenous injection into the patients.
The chances of rejection are low because patients’ own cells will be used and the physical strain from intravenous injection is limited, according to the team.
Examinees in the clinical trial must be aged between 20 and 64, and meet such conditions as having suffered their main injury in their cervical cord.
About 5,000 people in Japan suffer spinal cord injuries each year, which often causes loss of motor functions or sensory paralysis, but there is no effective therapy at present, according to Yamashita.
If nerves are regenerated through the stem cell-based therapy, patients may become able to move their hands or legs again.
Yamashita and Honmo told a press conference that the team has confirmed lasting effects of the method in basic research using mice.
In the coming trial, the cell preparation made from the mesenchymal stem cells will be injected into patients within 54 days of their spinal cord injury, they said.
Yamashita said the team hopes to finish the clinical trial as early as possible, but added that he cannot say at the moment when the therapy will be put into practical use.